The pH values of our body
Our body consists of 100 trillion cells, 29 organs (depending on whether they are categorized individually or in the organ network) and a double-digit number of body fluids. Each organ and body fluid has its own optimal pH.
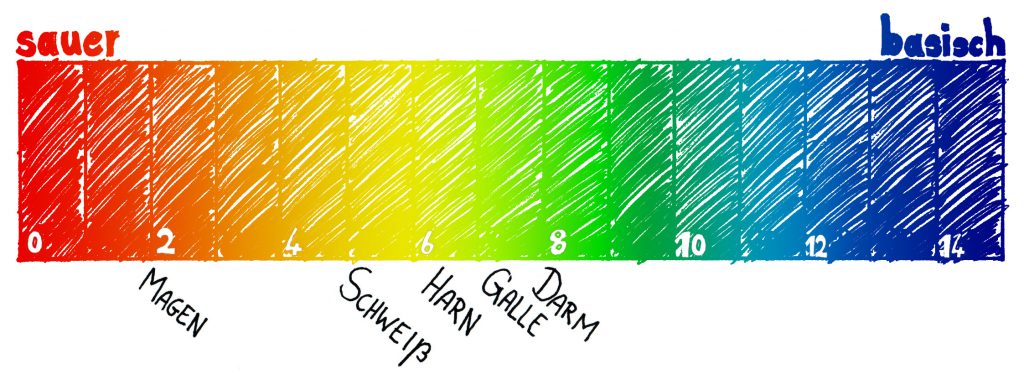
The abbreviation "pH" stands for the Latin "potentia hydrogenii" and represents the concentration of hydrogen ions in an aqueous solution. The more hydrogen ions are dissolved in a solution, the lower the pH value. Hydrogen ions have the property of certain vastly changing chemical components when you come into contact with them. The pH scale ranges from 0 to 14, where 0 denotes the strongest acidity and 14 the highest basicity. A pH of 7 is neutral.
The pH range goes from 1 (very acid) to 7 (neutral) to 14 (very basic). Each of our body fluids has its own optimal pH. When the pH values are not within limits, it can be dangerous to the body. Diseases have a more optimal environment, and it can even be life threatening, like if the blood is overly acidic. In other words, regulation of abnormal pH values can also lead to an improvement in health.
Here are some examples of PH values (approx. values):
Stomach: 1.2 to 3.0
Urine: 4.8 to 8.0
Sweat: 4.5 to 6.0
Muscles and organs: 6.9
Liver: 7,1
Saliva: 7.0 to 7.2
Bile: 6.5 to 8.2
Body cells: 7.0 to 7.3
Blood: 7.35 to 7.45
Pancreas: 8,0
Intestine: 8.0
PH values can ben measured through blood, saliva, or urine. A measurement of the blood pH value is performed by a physician in the form of a blood gas analysis. For regular checks, saliva measurements can be done, and urine ph values can be measured at home.
One can buy litmus strips in the pharmace. These react in color when they come into contact with liquids. A color scale can be used to determine the pH value, for example, of the urine.
If the measurement of the urine pH value is carried out several times a day, it will not always be the same value. Also, the time of the day plays a part (in the morning when the urine has been in the bladder for several hours, it is usually more acid than during the day / evening). A second major influencing factor is what we do with our bodies. When consuming coffee and other "sourmakers", the PH value decreases, and with consumption of apple cider, kaisernatron or high basic foods the levels will rise.
The pH of the urine has a wide with respect to fluctuations in the value. This tolerance window is significantly smaller at the blood pH. If the pH is below 7.35, the body contains too much acid. If the pH rises above 7.45, this is considered alkalosis.
Stomach: The lowest pH with 1.2-3 is stomach acid. The hydrochloric acid which is present is necessary in order to digest the food (especially proteins) and at the same time, kill the pathogens which are ingested from food or air. The stomach acid is so aggressive that the stomach would decompose itself were it not for the gastric mucosa. This also explains the massive complaints people have when the gastric mucosa is not intact.
Urine: with a value of 4.8 to 8.0, urine is clearly acidic (pH 4.8) and basic (up to pH 8.0). The urine is, of course, slightly acid since certain urinary stones form better in a basic environment than in an acidic environment. A slightly acidic urine is therefore absolutely correct and also important - under normal circumstances the acid with the urine from the body exits the body.
Sweat: The pH value of perspiration is slightly acidic at 4.5-6.0. Sweat is essentially a stable acid protection layer of the skin and provides a barrier against bacteria and fungi. Muscles and the cells of the organs have a pH value of about 6.9, a slightly more acidic value resulting from the fact that the cells are active around the clock and that acid is produced during the processing of our nutrients. The permanent activity (and acid production) must prevents a continuous deacidification so that the organism can survive. When the cells of our most important muscle - the heart muscle - are not deacidified and fall to a pH value of 6.2, the heart stops.
Liver: Secretions of the liver are slightly basic at pH 7.1. In the liver, urea synthesis takes place, which makes it possible for uric acid to be excreted through the urine. The liver therefore plays a large role in the acid-base balance.
Saliva: The value for saliva lies between 7.0 to 7.2, neutral to slightly basic. If the equilibrium of the PH value in the mouth is disrupted, this can damage the teeth over time as the acid attacks tooth enamel.
Gall Bladder: With its pH value of 7.5 to 8.2, bile increases the pH value of the acid coming from the stomach in order to prepare it accordingly for digestion in the intestine, thus also protecting the sensitive intestinal walls from burns.
Body Cells: Healthy body cells usually have a neutral to slightly basic pH between 7.0 to 7.3. In this value range, they reach their most stable state the maximum functional efficiency with the least energy expenditure. If this value falls, this can have far-reaching consequences which can reach to the uncontrolled cell division and dysfunction of the immune system.
Blood: The blood is in the basic range with a pH value of 7.35 - 7.45. During breathing, oxygen (O2) is absorbed into the lung, bound to red blood cells, and transported from the heart to the organs. There it is exchanged for carbon dioxide (CO2), transported back to the lung via the blood and released again during exhalation. An oxygen deficiency or an excess of carbon dioxide makes the blood "acid". The "acidic condition" tries to balance the body when the acid of the blood is intercepted. This "acid trap" ("buffer base") is the "bicarbonate" (hydrogen bicarbonate, HCO3-). The body also tries to restore the optimal acid-base ratio by means of a reflex-like exhalation and exhalation.
Pancreas: The secretion of the pancreas is markedly neutral with 8.0 in order to neutralize gastro-acidic food in the duodenum so that the nutrients of the organism can be absorbed in the small intestine.
Intestine: A pH value of 8.0 and higher is maintained in the intestine. If digestion caused by fermentation or rotting processes causes excessive acid formation in the intestine, the body regulates it by eliminating "disturbing" substances through diarrhea. Medically suppressing diarrhea is, in essence, not doing your body any good as what it is doing is basically getting rid of unhealthy elements.
- Nutrition (sugar, meat, coffee, alcohol)
- Stress and anger
- Smoking
- Shallow breathing (often caused by lack of activity)
- etc.
An over-acidification of the body or an imbalance of the acid-base balance can be the cause for many symptoms and diseases, not only for cancer, such as eczema and other allergies, autoimmune diseases, a disturbance of the intestinal milieu (which frequently accompanies a parasitic imbalance in the intestine.) So it makes sense - also preventively - to test your own pH from time to time and possibly by changing the your body’s “way of life.”
Healthy cells gain energy in different ways. One is called cell respiration. In the case of cellulitis, sugar, which we consume through food (in a pure form or as carbohydrate), is burned in the mitochondria, which act as the power center of a cell. A healthy cell is able to metabolize fats and proteins as energy. Mitochondrial respiration is referred to as aerobic energy production, since it is oxygen-dependent. Water (H20) and carbon dioxide (Co2) are formed.
Cancer cells are characterized by their mitochondria being defective / restricted in function. Instead, they gain their energy through anaerobic processes, also called fermentation. Sugar, which we all too often feed our bodies, is fermented with the production of lactic acid. Since cancer cells are dependent on sugar, they are significantly more sugar-rich than healthy cells. Using the PET-CT (invented by Otto Warburg), this is used to diagnose cancer cells.
Fermentation of sugar in the cancer cell produces lactic acid as a waste product, which makes the cell itself and the tissue around it acidic. Just as there is an optimal pH value in which healthy cells are particularly stable and maximally functional, it has a negative effect on the cells when the pH drops sharply, which is caused by the lactic acid of the cancer cell. The cells cannot survive in the acidic environment and die. For the cells of the immune system, this can mean that body-specific control programs such as apoptosis - programmed cell death - can no longer proceed. Defective cells are normally recognized and removed by apoptosis. Degenerate cells of immune cells can also be destroyed. If the pH value is too acidic, these processes can no longer proceed in a controlled manner. This condition makes it easier for tumors to spread, producing additional lactic acid.
With the knowledge about these processes, I asked myself the question how it could be that doctors and nursing staff did not clarify that you should eat sweets as little as possible, and that cravings for sweets can lead to the production of sugar-hungry cancer cells. Instead they always said: "Eat what you want, you need energy and can not lose weight, since sweets are helpful." In the morning I received a strong cell poison in the form of chemo, which was supposed to kill the cancer and while it was pouring into my veins, they served me pudding and cakes.
I sat with my very supportive friend and concluded that the logical consequence would be to raise the pH value of the body. We developed a strict, holistic as possible plan which was intended to help the body by means of the foods I ate daily, to make sure that its control and repair programs could work properly again. This plan and an explanation of all that it contained can be read here.